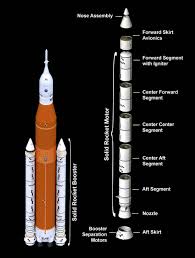
Liquid chemical propulsion
Liquid chemical propulsion exhibits two types namely; monopropellant and Bipropellant.
Bipropellants – as the name implies, this type of propulsion uses two liquid propellants. One as a
fuel(like liquid hydrogen l and the other as an oxidizer(liquid oxygen).
Some propellant combinations wont spontaneously combust on contact, they need an igniter just as a
car needs a spark plug to get started, this increases the complexity of the system. But not for so
long, because chemists came up with a combination that reacts on contact. These propellants are
called hypergolic because they don’t need a separate igniter. The combination of hydrazine plus
Nitrogen tetroxide is an example of hypergolic propellants. The Titan II, IIIB, IIIC, and IV NASA
rockets used this combination of propellants. Hypergolic propellants are stable at room temperature
for a very long time(months or even years). That is why they preferred for long term space missions,
other than the cryogenic propellants
Cryogenic propellants are stable at extremely low temperatures(about hundreds of degrees below zero,
centigrade), which is very difficult to fulfil by mission planners. However, cryogenic propellants
are more efficient and provide a higher performance compared to hypergolic propellants, since they
produce greater specific impulse
Monopropellant – as the implies, rough contact with a single propellant is used as the propellant.
These propellants are relatively unstable and easily decompose through contact with a suitable
catalyst. Hydrogen peroxide is a good example of a monopropellant. But the most used monopropellant
is hydrazine, because it readily decomposes when exposed to a catalyst and produces a specific
impulse of about 230s. Its short come is the toxicity.
Mars Reconnaissance Orbiter used a monopropellant propulsion system: there was fuel (hydrazine), but
no oxidizer. Thrust was produced by passing the fuel over beds of catalyst material just before it
entered the thruster, causing the hydrazine to combust.